Project Leaders
Pawel R Kiela
Principal Investigator
Albert Barberan
Co-Investigator
Paul Carini
Co-Investigator
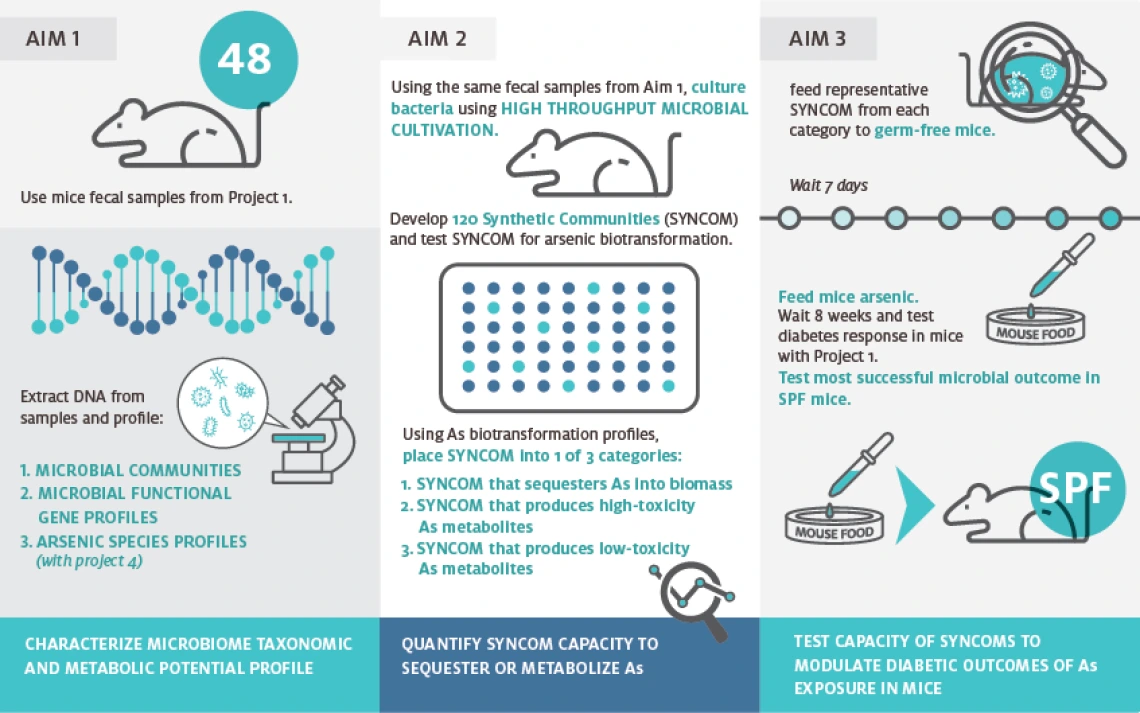
Summary
Legacy mine tailings that remain after extraction of economic metals are frequently enriched with co-occurring contaminants such as arsenic (As) that pose serious health hazards to neighboring communities and ecosystems. As ingestion has been associated with diabetes, numerous cancers, and cardiovascular disorders. The mode of action for As toxicity is not clear; however, the degree of toxicity is associated with the valence and the methylation state of As metabolites (e.g., trivalent As species, iAsIII, MMAIII, and DMAIII are two times more cytotoxic than iAsV; methylated pentavalent arsenicals are 10-fold less cytotoxic than AsV). The gut microbiome is a primary point of contact for As in the host because oral ingestion is the principal exposure route. In addition, in vitro studies have demonstrated the capacity of human colon microbiota to biotransform iAs to both more and less toxic forms. Thus, accurate As risk assessment requires understanding of presystemic contributions by the gut microbiome to the bioaccessibility and speciation of the host As load. The overall objectives of this project are to (1) contextualize the composition of the mouse gut microbiome with its functional capacity to metabolize As and (2) evaluate the capacity of defined As transforming microbial communities to affect in vivo diabetic outcomes following As exposure. The multidisciplinary team is employing a unique approach to identify specific associations between the composition of the gut microbiome, its genetic and functional capacity to sequester and/or transform As, and its capacity to either exacerbate or mitigate host diabetic outcomes in response to As exposure. Routine microbial taxonomic (16S rRNA gene amplicon profiling) and functional gene (shotgun metagenome) analyses are identifying the impact of host sex, age, and As exposure on mouse fecal community composition. This molecular analysis is being combined with function-based high throughput culture analysis of the same fecal communities to facilitate the design of 120 distinct synthetic microbial communities (SynComs). The functional capacities of each SynCom to transform/sequester iAs is being identified and will potentially capture emergent properties of microbially mediated As biotransformation that might be missed in studies using isolated phylotypes. The SynComs are being clustered in functional guilds with differing capacities to increase or decrease the As load experienced by the host. These SynComs are being tested in germ-free mice to evaluate the capacity of specific microbial consortia with distinct As biotransformation capacities to modulate diabetic outcomes of As exposure. It is hypothesized that microbial communities that reduce As toxicity and associated diabetic outcomes can be exploited as potential probiotics. This hypothesis is being tested through verification of the ability of positive outcome SynComs to colonize a specific pathogen free (SPF) mouse host and prevent or reduce pro-diabetic effects of iAsIII exposure.

