New Research Discovers Biological Mechanism Linking Chronic Arsenic Exposure to Cancer and Diabetes

A new study led by Dr. Donna D. Zhang, University of Arizona Superfund Research Center Associate Director, has identified the biological mechanism linking chronic arsenic exposure to diseases such as cancer, and Type 2 diabetes. These findings could result in potential new targets for drug development.
More than 34 million Americans have diabetes, according to the Centers for Disease Control and Prevention, and approximately 90-95% of them have Type 2 diabetes. One of the main risk factors is environmental toxicant exposure, particularly chronic exposure to arsenic, which has been shown to affect insulin production and sensitivity, blood sugar levels, and lipid profiles, all common features of diabetes onset and progression.
Because arsenic is a natural metalloid found in soil, it can be one of the most significant contaminants in drinking water globally, especially when ingested at unsafe levels. Arsenic is present in almost all groundwater sources in Arizona, particularly in rural areas. Combined with occupational exposures, such as mining, more than 160 million people worldwide are exposed to arsenic.
The study examined the effect of arsenic exposure on nuclear factor-erythroid 2 related factor 2 (NRF2) activation. NRF2 is a protein that plays an important role in maintaining cellular homeostasis, especially during times of oxidative stress when there is an imbalance of free oxygen radicals and antioxidants in the body.
Long-term oxidative stress, such as that caused by cigarette smoke, radiation, diets high in sugar, fat and alcohol, or environmental toxins, contributes to the development of a range of chronic conditions including cancer, diabetes, and neurodegenerative disease.
NRF2 is the body's governing regulator against oxidative stress. When the body enters an oxidative stress state, NRF2 is activated, and the process of cellular protection begins. When cellular homeostasis is restored, NRF2 levels return to normal.
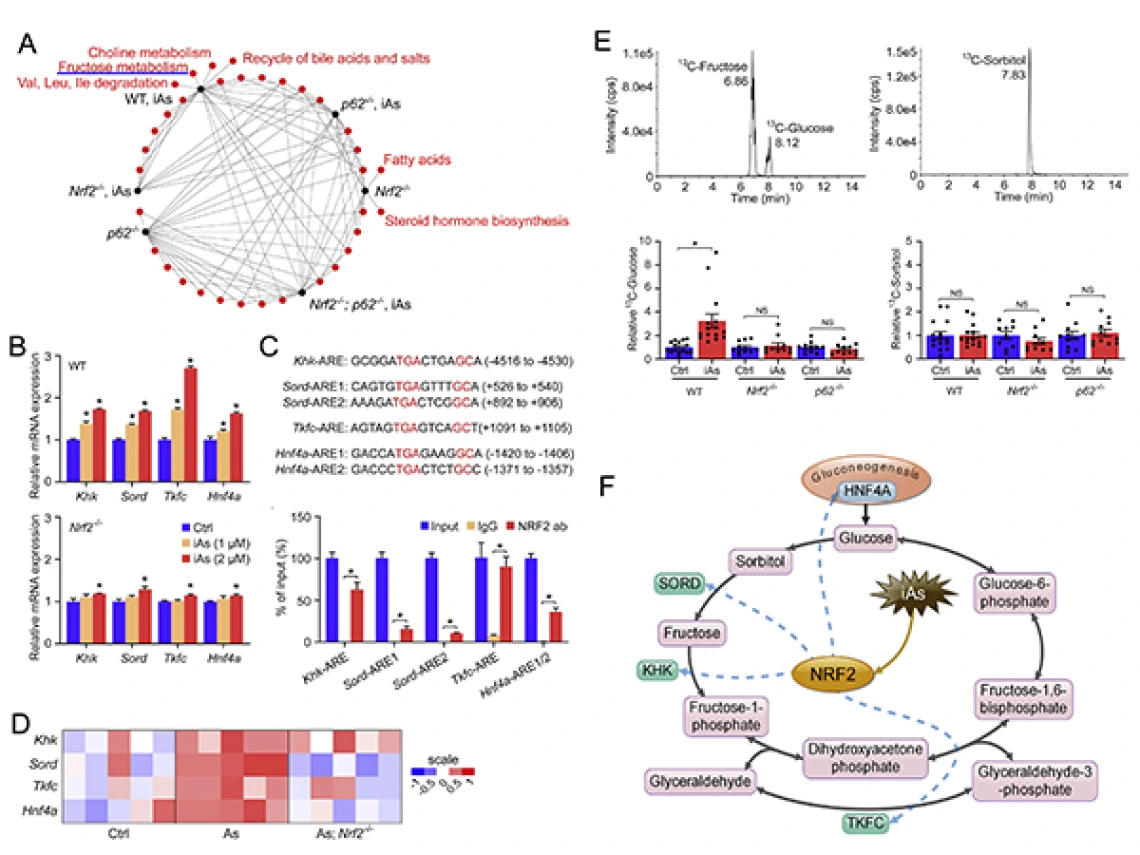
Dr. Zhang and the research team found that arsenic exposure results in the prolonged and uncontrolled activation of NRF2, which previously was determined to be a driver of cancer progression and resistance to anti-cancer therapy. In this study, they found that arsenic exposure resulted in glucose intolerance and decreased insulin sensitivity. In particular, prolonged NRF2 activation by chronic arsenic exposure caused shifts in pathways that control amino acid, fatty acid, carbohydrate, lipid and drug metabolism.
The findings demonstrated that prolonged NRF2 activation in response to arsenic increased glucose production in the liver and the release of that glucose to the bloodstream, which could represent a key driver of changes in systemic blood glucose.
Dr. Zhang said: “Hopefully this study will serve as a foundation for future toxicant-driven diabetes research here at the University of Arizona Health Sciences and elsewhere. Our eventual goal is to generate effective preventive or interventive strategies to treat exposed populations."
This story was modified and originally published by NEWS Medical Life Sciences.
Journal reference:
Liu P, Dodson M, Li H, Schmidlin CJ, Shakya A, Wei Y, Garcia JGN, Chapman E, Kiela PR, Zhang QY, White E, Ding X, Ooi A, Zhang DD. Non-canonical NRF2 activation promotes a pro-diabetic shift in hepatic glucose metabolism. Mol Metab. 2021 Sep;51:101243. doi: 10.1016/j.molmet.2021.101243. Epub 2021 Apr 30. PMID: 33933676; PMCID: PMC8164084.

