Project Leaders
Summary
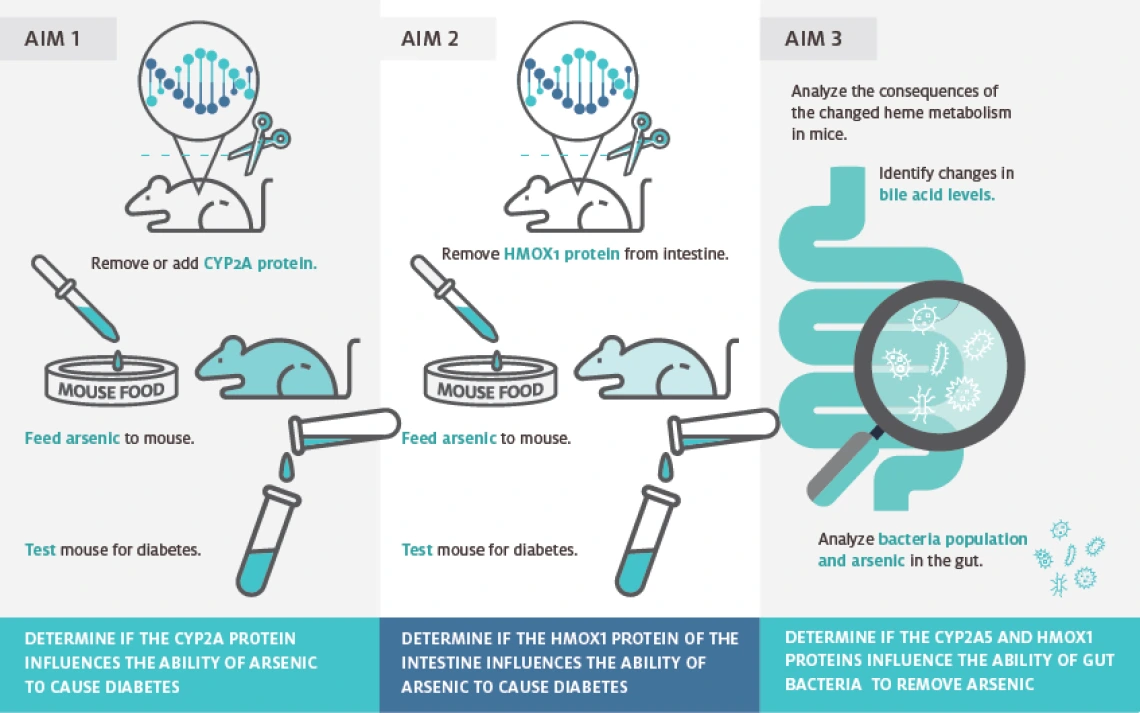
Environmental exposure to arsenic metalloids, the major toxic contaminants in mine tailings, is associated with increased incidence of metabolic diseases in humans, posing a significant health risk to people living near contaminated sites. Populations residing near mining sites may be exposed to mine tailings through the inhalation of dust particles or ingestion of contaminated water or food, as the arsenic contaminants may become bio-accessible and cause debilitating metabolic diseases such as diabetes. The diabetogenic potential of arsenicals in environmentally occurring mine tailing particulates is unclear, however, and the molecular mechanisms underlying the diabetogenic effects of arsenic are not fully understood – knowledge gaps that have markedly hindered disease prevention efforts. Nrf2, a master regulator of the antioxidant response, enhances cellular protective mechanisms through the canonic pathway, leading to activation of many antioxidant and biotransformation genes. Evidence also suggests, however, that Nrf2 may disrupt cellular homeostasis upon prolonged activation via a noncanonical pathway activated by the dysregulation of p62-dependent autophagy. This study is testing the novel hypothesis that Nrf2-stimulated protective mechanisms play an important role in limiting the adverse impact of chronic arsenic exposure through the modulation of host metabolic homeostasis and gut microbial arsenic biotransformation. Accordingly, the researchers believe that the disruptive events (studied in Project 1 - Diabetogenic Mine Tailings: Mechanistic Link Between Arsenic, NRF2, Autophagy, and Diabetes) and the protective mechanisms (this project) mediated by Nrf2 signaling are both important determinants of the diabetogenic risk of mine tailing arsenic metalloids. Nrf2 target genes include those for enzymes involved in the production or metabolism of bilirubin, including heme oxygenase 1 (Hmox1) and CYP2A (mouse Cyp2a5 and human CYP2A6). Strong clinical and experimental evidence indicates a protective role of bilirubin against the development of metabolic syndromes. Hmox1 was drastically induced in the intestine upon arsenic exposure in drinking water. Cyp2a5 was among the most highly and persistently induced genes in the mouse liver following chronic oral exposure to arsenic. These findings support the specific hypothesis that the induction of intestinal Hmox1 and hepatic CYP2A5/6 serves as an important counterbalance against the diabetogenic effects caused by chronic Nrf2 activation by mine tailing arsenic metalloids. The hypothesis is being tested through three specific aims. The outcome is expected to contribute to an integrated mechanistic view of the risk factors underlying arsenic's diabetogenic effects and thus fill an important knowledge gap that has hampered disease prevention efforts.



