Copper Mining and Processing: Processing Copper Ores
copper_processing_chart.png
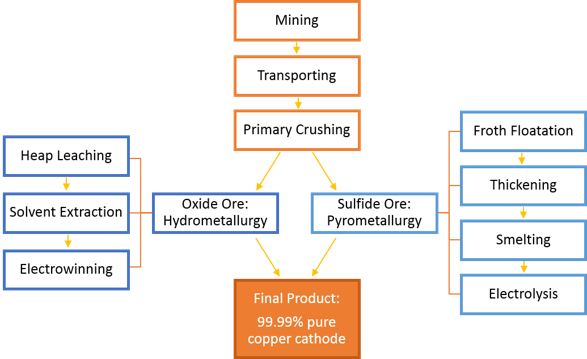
Copper processing is a complicated process that begins with mining of the ore (less than 1% copper) and ends with sheets of 99.99% pure copper called cathodes, which will ultimately be made into products for everyday use. The most common types of ore, copper oxide and copper sulfide, undergo two different processes, hydrometallurgy and pyrometallurgy, respectively, due to the different chemistries of the ore. Copper oxides are more abundant near the surface, but are considered low-grade ore, with a lower concentration of copper. Although this requires more ore to be extracted and processed, this process is less expensive, so oxides can still be mined at a profit. On the other hand, while copper sulfide ores are less abundant, they contain higher amounts of copper. Although the processing costs are higher, ultimately more copper can be extracted. Since each mine site is unique in its mineral composition, concentration, and quantities, the most economical and profitable processing of ore must be determined by the mine planners. When it is economically feasible, a mine may extract both types of copper minerals; when it is not possible, mines will only process either the copper oxides or the copper sulfides.
The first steps of copper processing are the same for both ores: mining and transporting. Copper mining is usually performed using open-pit mining, in which a series of stepped benches are dug deeper and deeper into the earth over time. To remove the ore, boring machinery is used to drill holes into the hard rock, and explosives are inserted into the drill holes to blast and break the rock. The resulting boulders are then ready for hauling; specialized haul trucks, conveyors, trains, and shuttle cars can all be used to haul the ore from the blasting site to the processing site. The size of the equipment needed to haul the tons and tons of ore is gigantic. Most ores are then sent through a primary crusher, which is typically located very close to or sometimes in the pit. This primary crusher reduces the size of the ore from boulder to golf ball-sized rocks.
A. Processing of Oxide Ore
Oxide ores are generally processed using hydrometallurgy. This process uses aqueous (water-based) solutions to extract and purify copper from copper oxide ores at ordinary temperatures, usually in three steps: heap leaching, solvent extraction, and electrowinning.
oxide_ore_processing.png
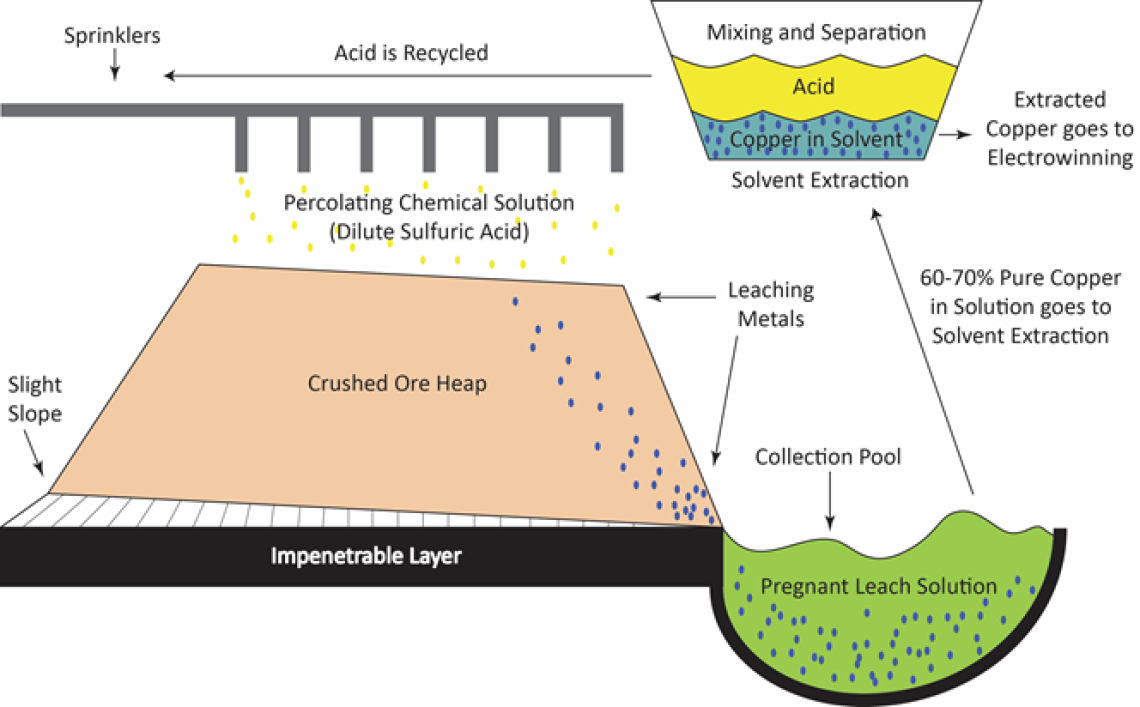
Heap Leaching is the process of using percolating chemical solutions to leach out metals. Heap leaching is very commonly used for low-grade ore, which would otherwise not be economical to send through a milling process. Following mining, transporting, and crushing to a consistent gravel or golf ball-size, the crushed ore is piled into a heap on top of an impenetrable layer, on a slight slope. The leaching reagent (dilute sulfuric acid) is sprayed through sprinklers on top of the heap pile and allowed to trickle down through the heap, where it dissolves the copper from the ore. The resulting “pregnant” leach solution of sulfuric acid and copper sulfate is collected in a small pool. The copper compound can now be seen at concentrations of between 60-70%.
The second step is solvent extraction, in which two immiscible (un-mixing) liquids are stirred and allowed to separate, causing the cooper to move from one liquid to the other. The pregnant leach solution is mixed vigorously with a solvent. The copper migrates from the leach solution into the solvent. The two liquids are then allowed to separate based on solubility, with copper remaining in solution in the solvent, and impurities remaining in the leach solution. The leftover leach solution is then recycled, by adding additional acid and sending it back to the sprinklers in the heap leaching process.
electrowinning.jpg
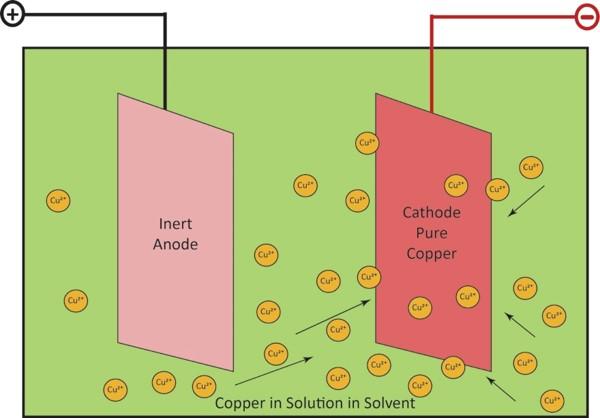
The last step is called electrowinning, a type of electrolysis. An electrical current passes through an inert anode (positive electrode) and through the copper solution from the previous step, which acts as an electrolyte. Positively-charged copper ions (called cations) come out of solution and are plated onto a cathode (negative electrode) as 99.99% pure copper.
B. Processing of Sulfide Ore
Sulfide ores are generally processed using pyrometallurgy, the extraction and purification of metals by processes involving the application of heat. This process uses a series of physical steps and high temperatures to extract and purify copper from copper sulfide ores, in four basic steps: 1) froth flotation, 2) thickening, 3) smelting, and 4) electrolysis.
Following mining, transporting, and crushing to a consistent gravel or golf ball-size, the crushed ore is further processed at a mill using secondary crushers, and reduced to pebbles, and finally to fine sand. After the copper ore is crushed, liquid is added to make it a slurry. The slurry is a mix of valuable copper ore minerals and “worthless” rock, called gangue (pronounced “gang”). The slurry is placed in a tank and a process called froth floatation is used to separate the copper minerals from the gangue. Chemical reagents called “collectors” are added to the slurry and bind to the copper particles, making them hydrophobic, or waterproof. Pipes are used to blow air into the bottom of the tank to create bubbles, which rise to the surface, taking the waterproof copper sulfide particles along. The froth of copper-rich bubbles at the top of the tank is then skimmed off for further processing. The gangue sinks to the bottom of the tank to be removed or disposed of as mine tailings.
The next stage after froth flotation is the thickening stage. The froth is poured into large tanks called thickeners. The bubbles break and solids from the froth solution settle at the bottom of the tank. The solids are then filtered to remove excess water, which can be reused in processing additional batches of sulfide ore. The final product of the thickening stage is a combination of 30% copper and other metals; this copper concentrate is then sent to the smelter.
anode_slabs.png

At the smelter, high temperatures are used to further purify the ore in a series of smelting steps. The copper concentrate is first sent through the smelting furnace to be heated up to 2,300 °F and converted into molten liquid. The heated liquid is poured into a slag-settling furnace. This step produces a combination of matte, a mixture of copper, sulfur and iron, and slag, a dense, glassy material made of iron, silica, and other impurities. The copper matte created by the smelting furnace contains 58-60% copper. The molten matte is then taken to another furnace called a converter to have the remaining iron and sulfur burned off; the product is referred to as blister copper, which contains 98% copper, and taken to the anode smelter. The blister copper is yellow; when the oxygen in the copper is burned off in the anode smelter, it turns a blue-green color. The resulting product, molten anode copper, is poured into molds called anode-casting wheels. The cooled anode slabs are 99% pure copper, are now copper-colored, have two handles molded on top, and are two inches thick, three feet wide, three-and-a-half feet high, and weigh 750 pounds.
copper_electrolysis.png
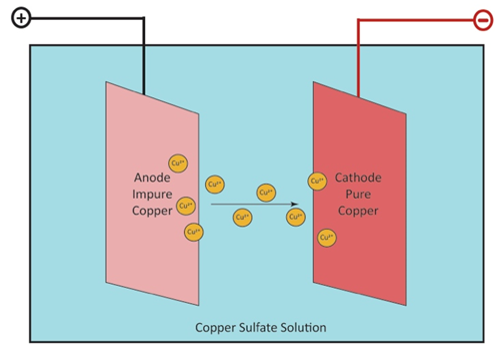
The copper anode slabs are then refined in a final step called electrolysis. The anode slabs are hung in a large tank full of an electrolyte solution made of copper sulfate and sulfuric acid. Thin sheets of pure copper, which are called cathodes and weigh about 15 pounds each, are hung in between the anodes. An electric current is applied, and positively-charged copper ions (called cations) leave the anode (positive electrode) and move in solution through the electrolyte solution to be plated on the cathode (negative electrode). Other metals and impurities also leave the anode to drop to the bottom of the tank or stay in the electrolytic solution. These impurities are collected and may be refined to recover other metals such as silver and gold. After 14 days of electrolysis, the anodes have gradually disappeared, and the copper cathodes now weigh 375 pounds each and contain 99.99% pure copper. The cathodes are taken out of the tank and rinsed with water to prevent further reaction. The finished copper cathodes can then be made into wires, plates, tubes, and other copper products.
C. Recycling Copper
In addition to processing copper ores, new and old copper scrap or copper alloys can be melted, re-purified, and recycled into new components. It is estimated that such recycling supplies 50% of copper used in the copper industry (Scott, 2011). In 2010, 770,000 metric tons of copper were recycled, at an estimated value of nearly six billion dollars (Papp, 2010).

